Research Overview
Aging is a complex phenotype associated with a decline in health and an increased probability of death. There are multiple visible aging effects at the organismal level in terms of health and disease, and the diversity is overwhelming. Importantly, the molecular changes that drive aging are less diverse, irrespective of the tissue or organ that is affected. Hence, improving the negative effects of aging may be more feasible at the molecular, rather than the organismal, level. The aim of our research is to better understand the primary molecular changes, or hallmarks, that occur during the processes of cellular senescence and aging. We are particularly interested in the regulation of telomeres, DNA mutation rates and the preservation of proteostasis during aging and senescence. We hope that a complete mechanistic understanding of these processes will lead to increased overall healthspan.
1. Telomere length regulation and replicative senescence
Every time a cell divides the chromosomes get shorter and shorter due to telomere attrition. This phenomenon is referred to as the “end replication problem” and it affects all organisms with linear chromosomes. When telomeres become very short, they prevent cells from further proliferation and induce a state of senescence. Senescence induced by telomere shortening is referred to as replicative senescence. Replicative senescence is an important feature as it prevents uncontrolled cell proliferation and can therefore be considered as a tumor suppressor. The tumor suppressor feature of senescence is beneficial in younger organisms to keep them cancer free, however the continual accumulation of senescent cells over time can drive organismal aging and age-related disease. Hence, it is critical to tightly regulate telomere shortening rate in order to 1. allow replicative senescence to function as an anti-cancer mechanism, but 2. prevent senescence from occurring in a accelerated manner, which would drive pre-mature aging.
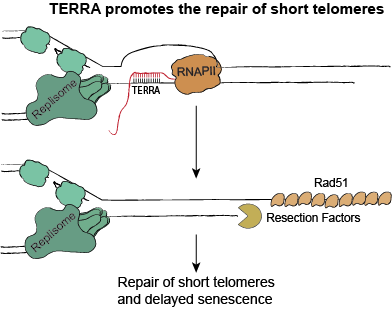
We have recently shown that the non-coding RNA, TERRA, is important for controlling telomere length during the senescence process. TERRA forms RNA-DNA hybrids (R-loops) at short telomeres and promotes telomere repair (see diagram on right). In this manner, TERRA prevents cells from entering senescence too quickly. We are trying to understand, in complete detail, the molecular regulation of TERRA and R-loops at telomeres. We are performing these experiments in yeast as the regulation of TERRA and R-loops is similar to what occurs in human cells. Our long-term goal is to be able to manipulate TERRA levels to control rates of senescence. This may have important implications for telomere biology disorders, where shortened telomeres result in premature aging phenotypes.
Publications related to the project (from our lab):
- Bento F, Longaretti M, Borges Pires V, Lockhart A and Luke B (2025) RNase H1 and Sen1 ensure that transient TERRA R-loops promote the repair of short telomeres. EMBO Rep, 26:3032-3044.
- Graf M*, Bonetti D*, Lockhart A*, Serhal K, Kellner V, Maicher A, Jolivet P, Teixeira MT and Luke B (2017) Telomere length determines TERRA and R-loop regulation through the cell cycle. Cell, 170:72–85.
- Balk B*, Maicher A*, Dees M, Klermund J, Luke-Glaser S, Bender K and Luke B (2013) Telomeric RNA-DNA hybrids affect telomere-length dynamics and senescence. Nat Struct Mol Biol, 20:1199–1205.
2. Transcription-replication conflicts and mutagenesis during aging - The role of RNase H enzymes
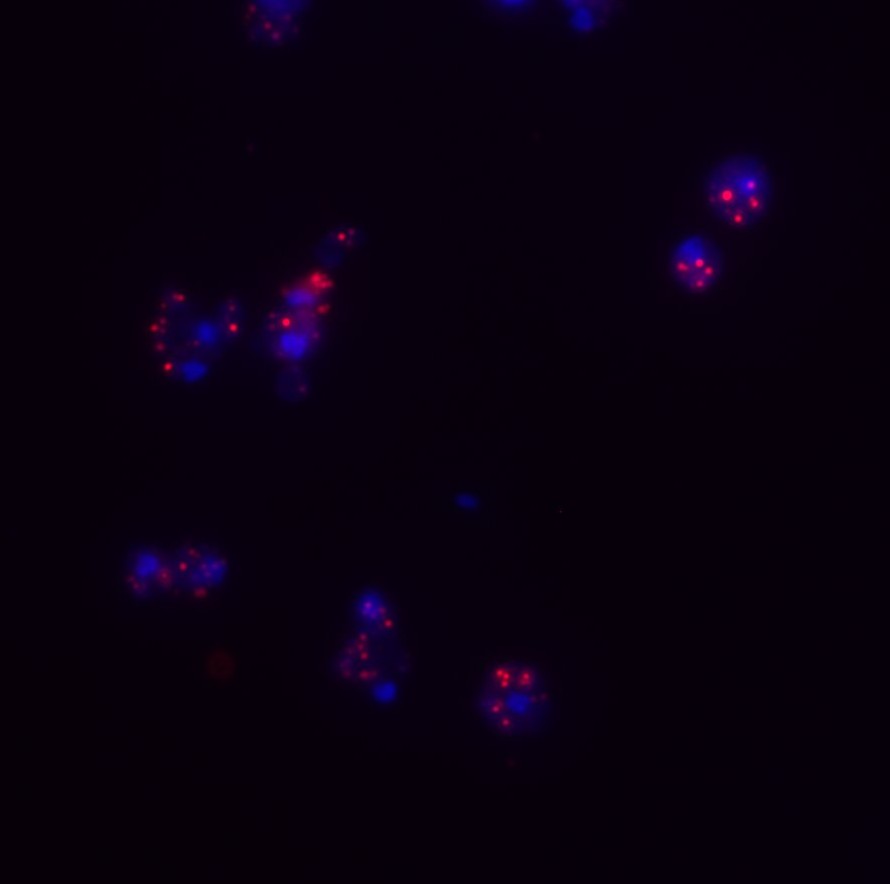
As we age, multiple intra-cellular functions are compromised. Interestingly, it has recently been shown that the speed of transcription increases in older, compared to younger, organisms. The alteration of transcription rates has multiple knock-on effects including increased torsional stress, altered transcription termination usage and the increased probability of an unscheduled interaction between the transcription machinery and the DNA replication machinery; a so called transcription-replication conflict (TRC). TRCs also lead to the formation of RNA-DNA hybrids and R-loops (red foci in figure on right). Hence, it is important to understand how TRCs are regulated in both normal and aged cells so that this type of genomic stress can be managed. We have shown that when TRCs accumulate, the RNase H1 enzyme gets activated and its protein levels increase. RNase H1 is an enzyme that is devoted to the removal of RNA-DNA hybrids and we are currently trying to understand how its regulation is controlled.
Furthermore, we are using RNA polymerase II mutations with altered rates of transcription and have demonstrated that they accumulate RNA-DNA hybrids. We are trying to understand which R-loop resolvases get activated when transcription speed changes. Our hypothesis is that this regulation will have important implications for aging biology. We have shown that increasing transcription speed is sufficient to decrease lifespan in yeast.
Finally, we are trying to understand how, and why, mutations in the DNA increase as we age, as this is thought to play an important role in the development of aging diseases and aging phenotypes. We have now established that topoisomerase I (Top1) causes mutations in aged cells at an increased rate. This increased mutation frequency may be related to the altered rates of transcription and torsional stress, but they may also be linked to rNMP incorporation into the genome.
Publications related to the project (from our lab):
- Wagner CB, Longaretti M, Sergi SG, Singh N, Tsirkas I, Bento F, Wong RP, Wilkens M, Hamperl S, Butter F, Aharoni A, Ulrich HD and Luke B (2025) Rad53 regulates RNase H1, which promotes DNA replication through sites of transcription-replication conflict. Cell Rep, 44:116565.
- Schindler N*, Tonn M*, Kellner V, Fung JJ, Lockhart A, Vydzhak O, Juretschke T, Möckel S, Beli P, Khmelinskii A and Luke B (2023) Genetic requirements for repair of lesions caused by single genomic ribonucleotides in S phase. Nat Commun, 14:1227.
- Lockhart A, Pires VB, Bento F, Kellner V, Luke-Glaser S, Yakoub G, Ulrich HD and Luke B (2019) RNase H1 and H2 are differentially regulated to process RNA-DNA hybrids. Cell Rep, 29:2890–2900.e5.
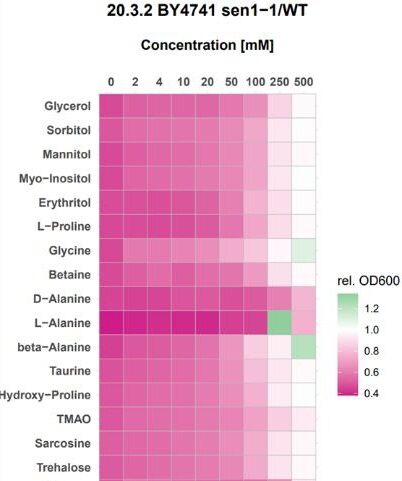
3. The effects of osmolytes on proteostasis to prevent age-associated disease
Recently, it has been demonstrated that the osmolyte, taurine, can extend lifespan in a wide range of organisms when administered as a supplement. Furthermore, many age-related ailments were improved when increasing amounts of Taurine were given to mice. Despite these encouraging results, it is not fully understood how taurine works on the molecular level to extend both lifespan and healthspan. We used yeast as a model organism to interrogate the effects of taurine on the 5 primary molecular hallmarks of aging. Taurine did not affect telomere length, DNA repair, autophagy regulation or epigenetic gene regulation, however it did improve proteostasis. We have shown that taurine maintains proteins in a folded and functional state when they are challenged with stresses, such as heat, age and mutation. We are investigating the possibility of using taurine, and other osmolytes, in combination to protect against aging and other diseases associated with protein misfolding. We have modelled Senataxin mutations that cause ALS4 and AOA2 in humans onto the yeast Sen1 protein and shown that taurine and other osmolytes can rescue the associated phenotypes (see figure on right).